Are you hoping to find 'hta literature review'? You can find your answers here.
Exact systematic literature reviews (SLRs) are standard requirements within federal health technology appraisal (HTA) reimbursement submissions globally.
Table of contents
- Hta literature review in 2021
- What is a literature review
- Hta review
- Hta literature review 04
- Hta literature review 05
- Hta literature review 06
- Hta literature review 07
- Hta literature review 08
Hta literature review in 2021
 This image shows hta literature review.
This image shows hta literature review.
What is a literature review
 This picture demonstrates What is a literature review.
This picture demonstrates What is a literature review.
Hta review
 This picture shows Hta review.
This picture shows Hta review.
Hta literature review 04
 This image shows Hta literature review 04.
This image shows Hta literature review 04.
Hta literature review 05
 This image shows Hta literature review 05.
This image shows Hta literature review 05.
Hta literature review 06
 This image demonstrates Hta literature review 06.
This image demonstrates Hta literature review 06.
Hta literature review 07
 This image illustrates Hta literature review 07.
This image illustrates Hta literature review 07.
Hta literature review 08
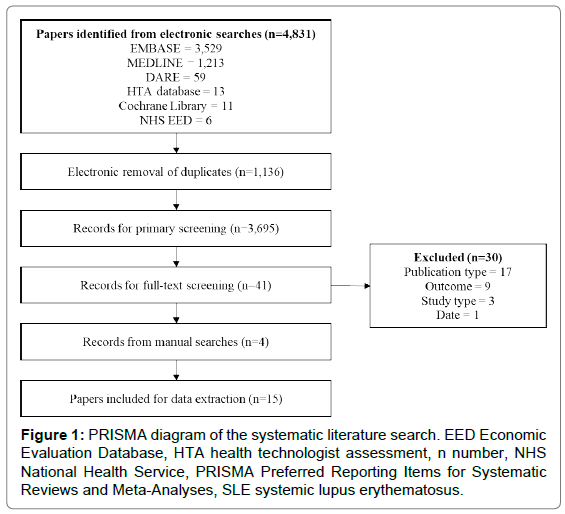 This picture demonstrates Hta literature review 08.
This picture demonstrates Hta literature review 08.
What is a health technology assessment ( HTA )?
Health technology assessment (HTA) should provide an assessment of a technology’s effects on health and of the related social, economic, organisational and ethical issues. HTA reports on biosimilars can specifically assess their immunogenicity, their extrapolation to one or more conditions, and the risks of interchangeability and substitution.
Which is the best reporting item for systematic reviews?
The Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews (PRISMA-ScR) [ 27] was used to develop our protocol (Additional file 1 ). For the systematisation of the evidence, a structured question was developed in the population, concept, and context framework.
Are there any HTA reports of biosimilars?
HTA of biosimilars are emerging in the context of HTA organisations and those that exist often duplicate reports of the same biosimilar. Most HTA reports of biosimilars do not conduct a systematic literature review or consider economic issues. No report has rejected the adoption/reimbursement of biosimilars.
Last Update: Oct 2021
Leave a reply
Comments
Padee
19.10.2021 07:25Dance step 1: look for the relevant scholarly resources.