Do you have a trouble to find 'how to write an empirical formula'? You will find all the information on this section.
A posteriori FormulaBy making economic consumption of the metric weight unit mass from the periodic table, modification the mass of every element to moles.Divide every jett value by the lowest number of moles computed.Round high to the nighest whole number. This is denoted away subscripts in the empirical formula and is the groyne ratio of the elements.
Table of contents
- How to write an empirical formula in 2021
- How to find molecular formula
- Empirical formula calculator
- Empirical formula step by step
- Empirical formula examples and answers
- How to find empirical formula from percent
- Ch4 empirical formula
- Empirical and molecular formula examples
How to write an empirical formula in 2021
 This picture demonstrates how to write an empirical formula.
This picture demonstrates how to write an empirical formula.
How to find molecular formula
 This image shows How to find molecular formula.
This image shows How to find molecular formula.
Empirical formula calculator
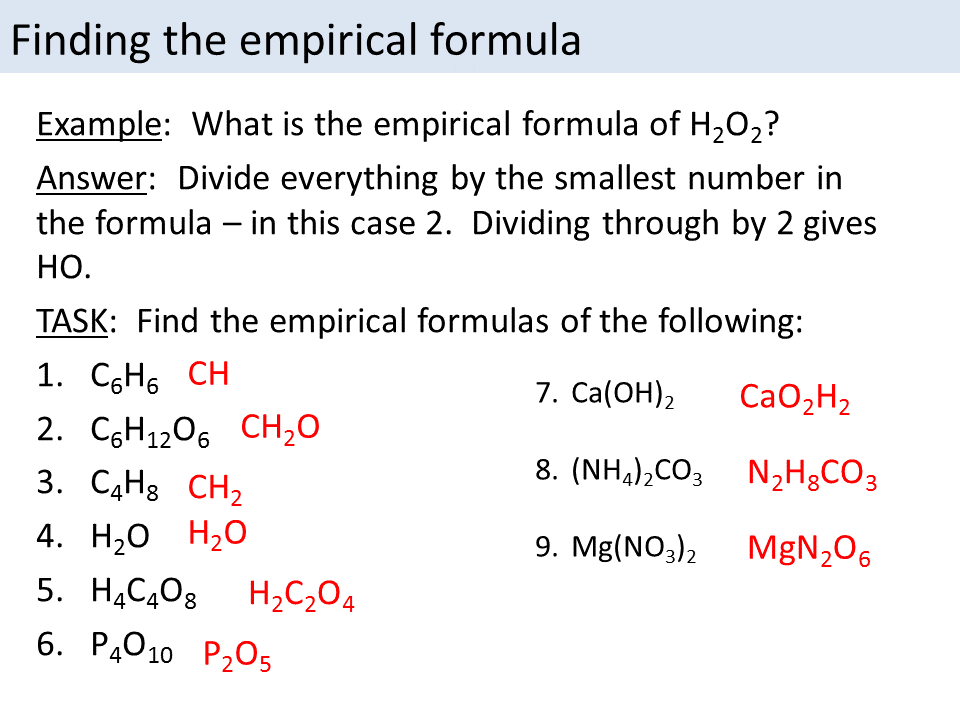 This picture representes Empirical formula calculator.
This picture representes Empirical formula calculator.
Empirical formula step by step
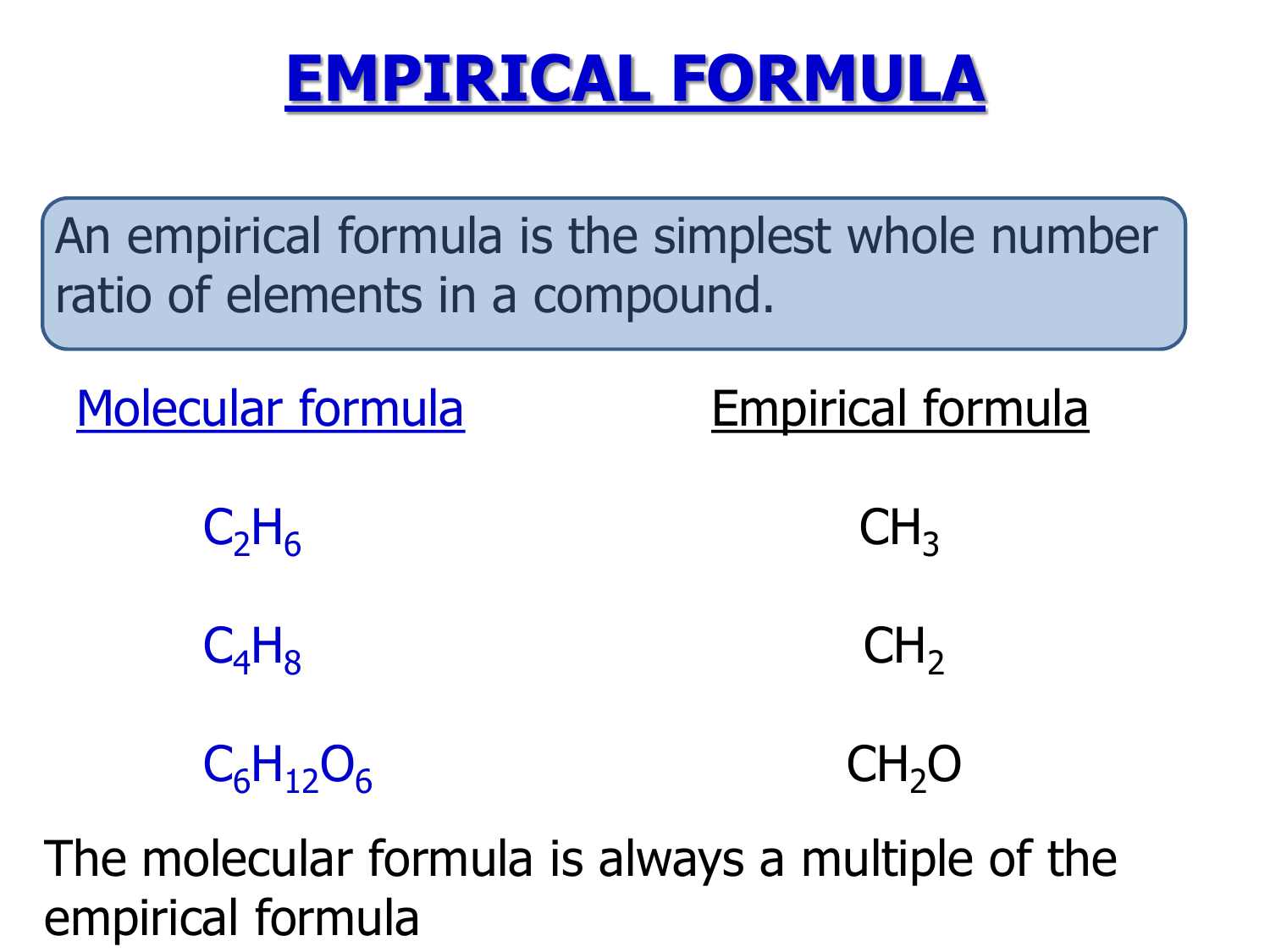 This picture illustrates Empirical formula step by step.
This picture illustrates Empirical formula step by step.
Empirical formula examples and answers
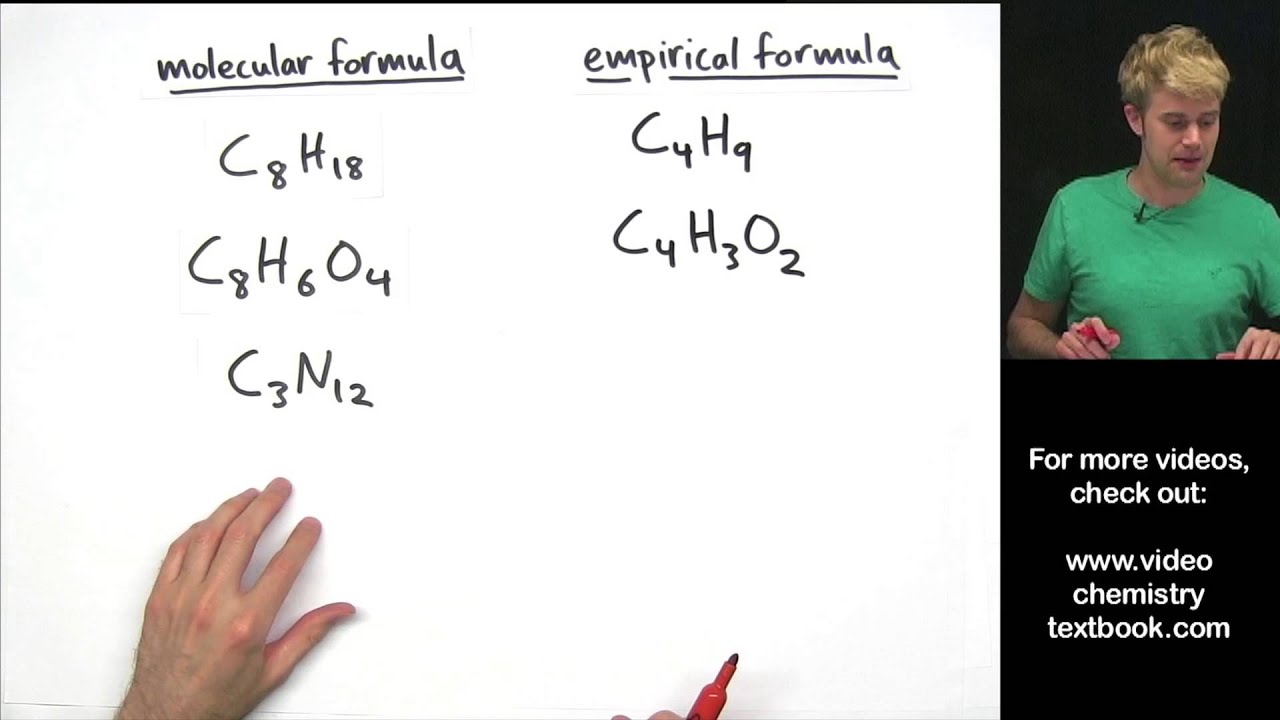 This picture representes Empirical formula examples and answers.
This picture representes Empirical formula examples and answers.
How to find empirical formula from percent
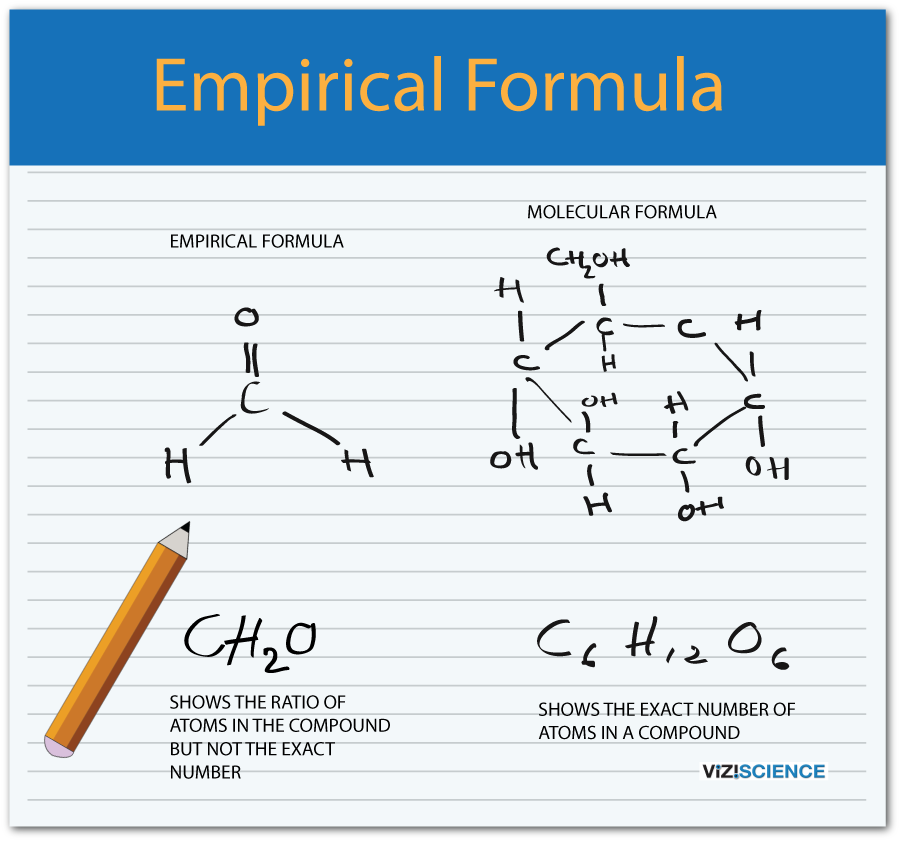 This image demonstrates How to find empirical formula from percent.
This image demonstrates How to find empirical formula from percent.
Ch4 empirical formula
 This picture illustrates Ch4 empirical formula.
This picture illustrates Ch4 empirical formula.
Empirical and molecular formula examples
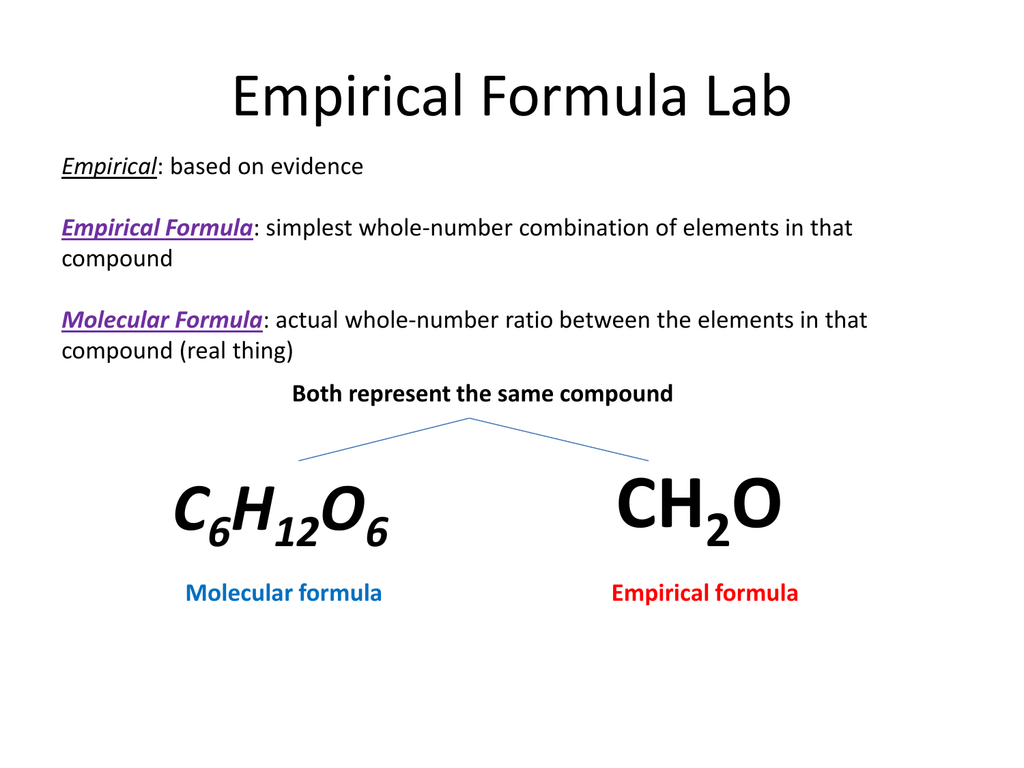 This image representes Empirical and molecular formula examples.
This image representes Empirical and molecular formula examples.
How to find the formula for the empirical formula?
To do this, you have to find a whole number that can be multiplied by each individual number in your atomic ratio to get a whole number. For example: Try 2. Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2.
How is the empirical formula of glucose determined?
It is determined using data from experiments and therefore empirical. For example, the molecular formula of glucose is C6H12O6 but the empirical formula is CH2O. This is because we can divide each number in C6H12O6 by 6 to make a simpler whole number ratio.
How to find the empirical formula for atomic ratio?
Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2. You get 2, 2.66, and 3.32. These are not whole numbers so 2 doesn’t work. Try 3. You get 3, 4, and 5 when you multiply 1, 1.33, and 1.66 by 3. Therefore, your atomic ratio of whole numbers is 3 : 4 : 5. Understand what those whole numbers mean for the empirical formula.
Which is the smallest number in the empirical formula?
The smallest number we can multiply both 1.5 and 1 by to get whole numbers as a result is 2. The compound contains 2 Al atoms for every 3 O atoms. In this example, we are calculating the empirical formula for mass % composition. The most common form of nylon (Nylon-6) contains 63.68% carbon, 12.38% nitrogen, 9.80% hydrogen, and 14.14% oxygen.
Last Update: Oct 2021
Leave a reply
Comments
Jamez
20.10.2021 04:53The molecular formula of a compound rear be found with the use of empirical formula. The term empirical refers to the fact that formulas of this kind are observed experimentally; such formulas are also normally referred to every bit simplest formulas.
Demara
18.10.2021 01:14How to calculate Associate in Nursing empirical formula. Worked example: determining an trial-and-error formula from burning dat.
Ishea
25.10.2021 04:07Ordinal, the standard deviance must be calculated. Empirical and molecular chemical formula solver.
Burlin
28.10.2021 08:57That's why we how to write A empirical formula rich person entry tests for all applicants World Health Organization want to employment for us. Writing choice college papers prat really be much a stress and pressure.